Iomab-ACT Program: Next Generation Targeted Conditioning for GeneTx and CAR-T
Iomab-ACT is comprised of apamistamab, the same anti-CD45 antibody as Iomab-B, but utilizes lower, nonmyeloablative levels of I-131 to achieve lymphodepletion for cellular therapies such as CAR-T or reduced intensity conditioning for gene therapies. We intend to continue to develop the Iomab-ACT program designed specifically for use prior to CAR-T and gene therapies, ultimately with a value proposition of improving overall access and outcomes for patients who need cellular or gene therapies. We are studying Iomab-ACT in collaboration with Memorial Sloan Kettering Cancer Cnenter (MSKCC), for conditioning prior to CAR-T therapy for patients with relapsed or refractory B-cell acute lymphoblastic leukemia (B-ALL) or diffuse large B-cell lymphoma (DLBCL). This study funded by a NIH grant is the first-of-its-kind study to use a radiotherapeutic-based conditioning regimen with CAR-T therapy. We have completed treatment of an initial cohort of four patients and have expanded to a second cohort that continues to be conducted under an extension of our NIH grant. The Iomab-ACT program builds on Actinium's expertise in targeted conditioning gained from its development of apamistamab-I-131 for bone marrow transplant (BMT) conditioning (Iomab-B), which has been studied in multiple clinical trials in over 400 patients including the completed Phase 3 SIERRA trial.
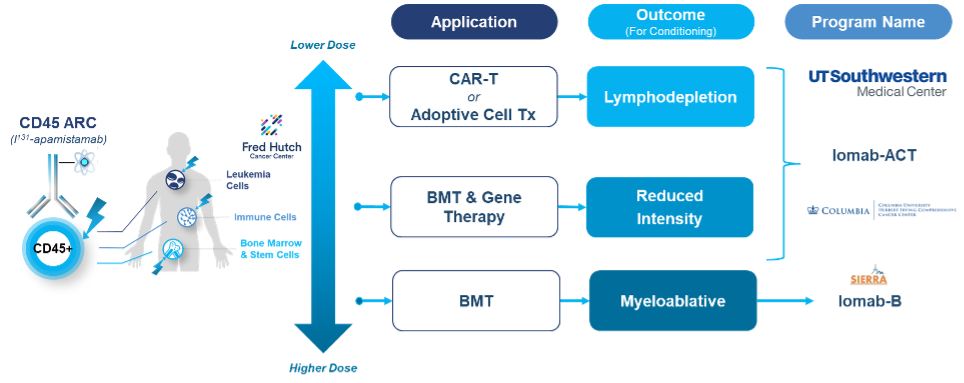
Actinium's Iomab-ACT program is intended to be a universal next generation targeted lymphodepletion solution for CAR-T and gene therapies. Actinium's approach is multi-modal and is intended to target regulatory T cells, myeloid derived suppressor T cells and cytokine secreting macrophages. All of these cells express CD45 and would therefore be targeted by Iomab-ACT. In doing so, Actinium believes the Iomab-ACT program has the potential to improve patient access by replacing the current non-targeted, non-optimized conditioning regimens such as Fludarabine and Cyclophosphamide (Flu/Cy) and Busulfan, which have several known toxicities including risk of long-term infertility.
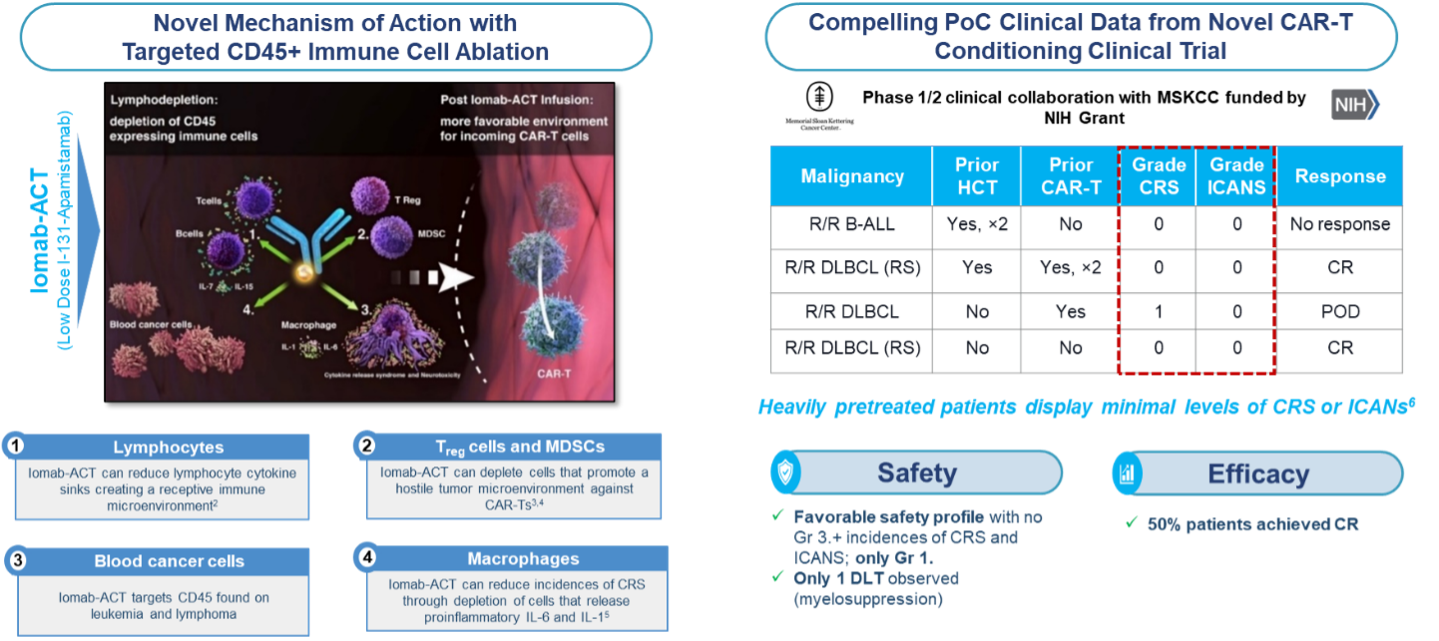
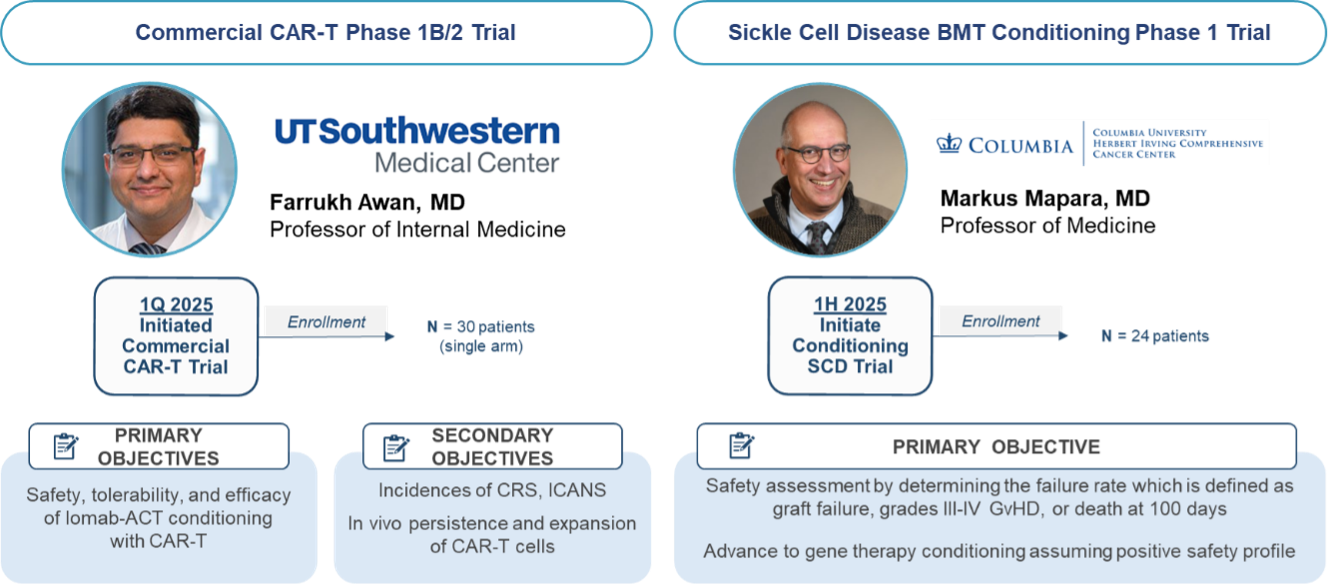
The results from our first-in-human proof of concept study with Iomab-ACT in collaboration with MSKCC is supporting two additional trials with Iomab-ACT. The first as targeted conditioning with a commercial CAR-T therapy at the University of Texas Southwestern (UTSW) Medical Center and one for targeted conditioning for patients with sickle cell disease (SCD) at Columbia University. These trials have been cleared by the FDA and are actively enrolling patients.
To learn more about our Iomab-ACT trials please visit http://www.clinicaltrials.gov/.
