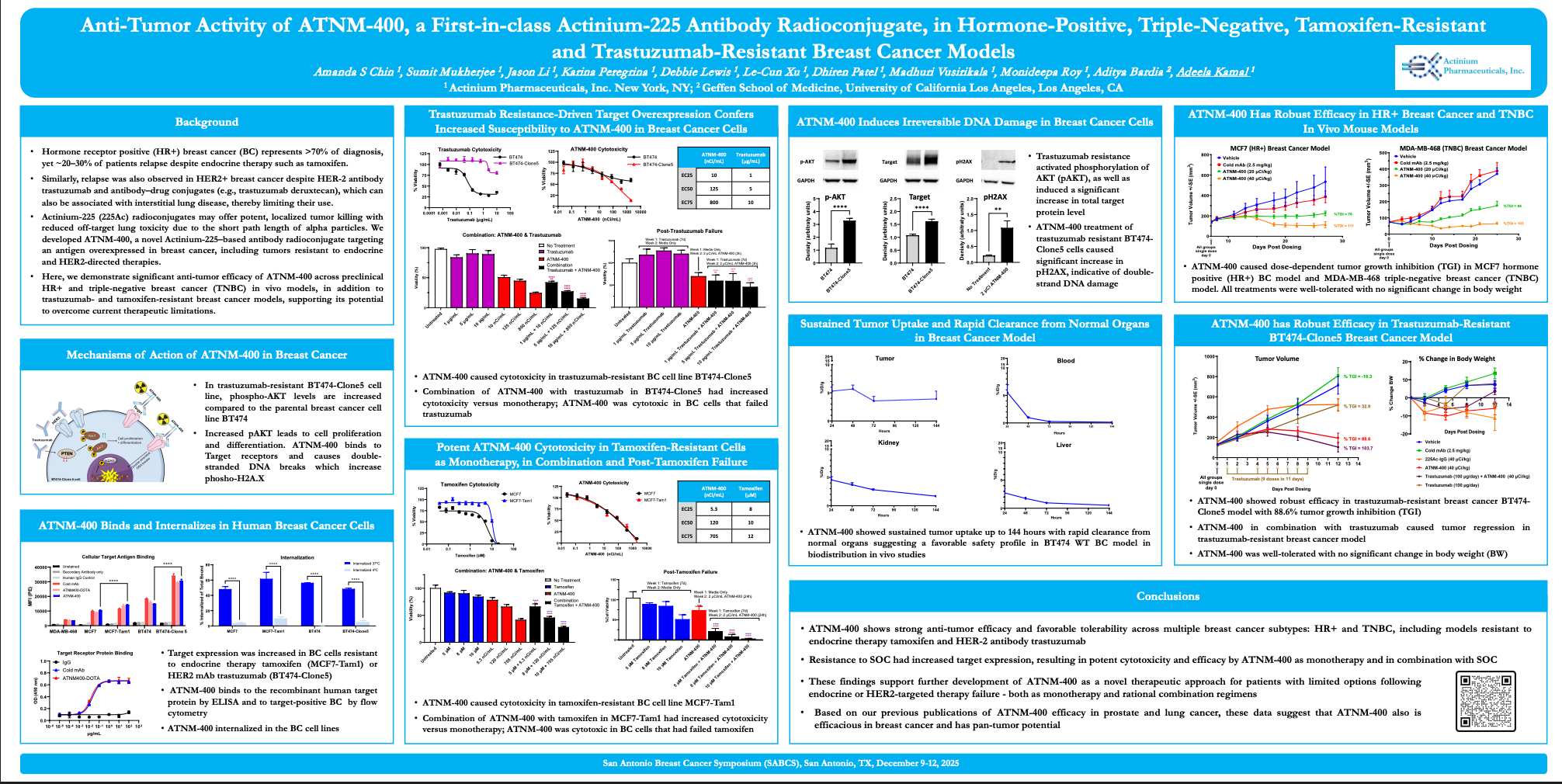
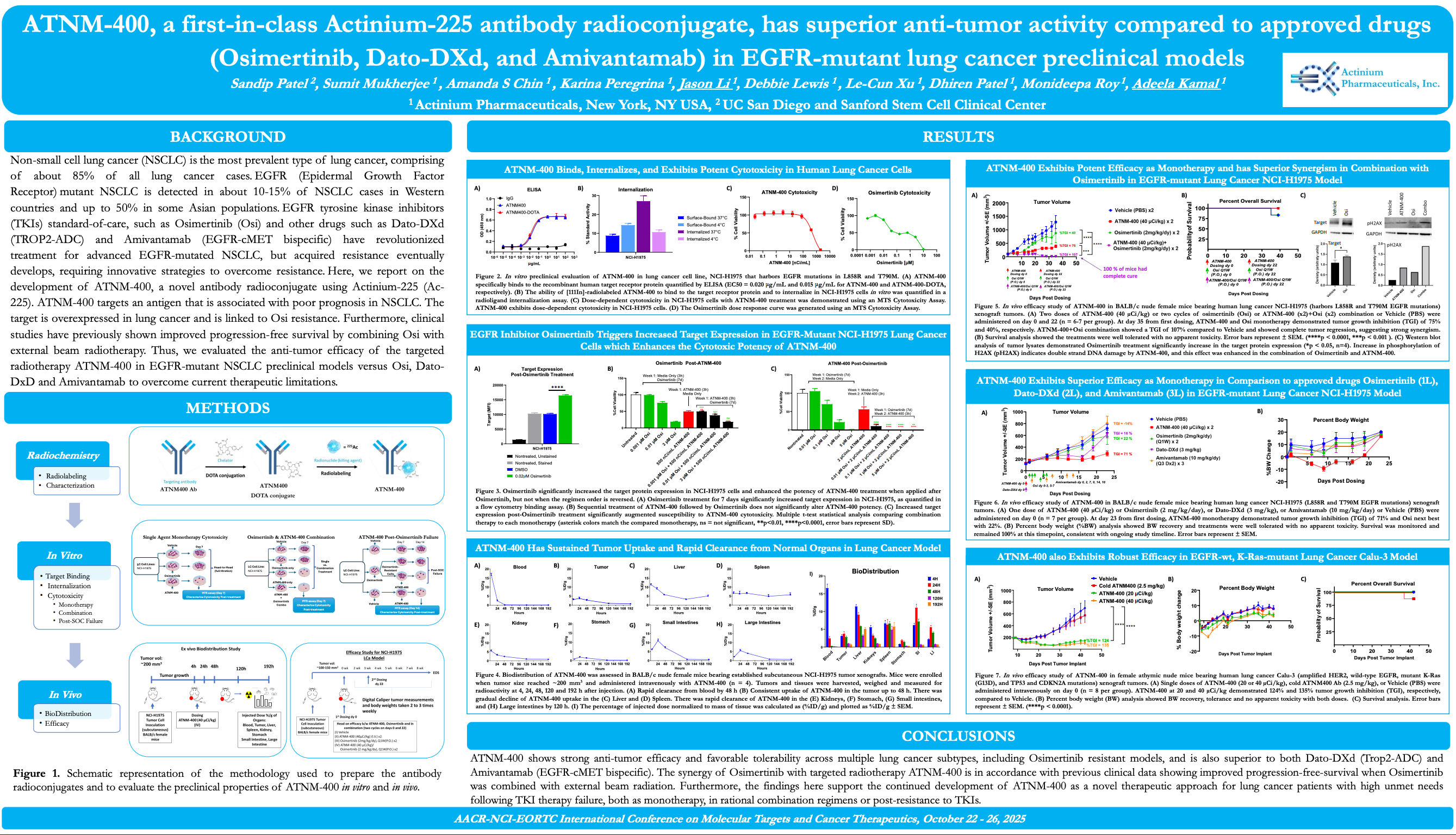
R&D Technology & Platform
Actinium's Antibody Warhead Enabling (AWE) Technology Platform
Actinium's AWE technology platform is used to produce ARCs or Antibody Radiation Conjugates, a highly potent and selective form of targeted radiotherapy. ARCs enable the precision targeting of the proven therapeutic power of radiation to tumors and its synergistic potential with other therapeutic modalities that cannot be matched by traditional external beam radiation, cytotoxic chemotherapy or biologic therapies. AWE enabled ARCs exploit the use of highly selective targeted biological agents such as monoclonal antibodies that can seek out and bind cancer antigens found on the tumor cell surface to deliver potent radioisotopes that are capable of producing double strand DNA breaks for which there is no known resistance or repair mechanisms.

Figure: Antibody Radio-Conjugates (ARCs) comprised of a monoclonal antibody, linker and radioisotope
Through our AWE technology platform we are able to utilize multiple radioisotopes to match the needs of the desired indication. We have demonstrated our ability to label both hematologic and solid tumor targeting agents with isotopes ranging from beta-emitters Iodine-131 (I-131) and Lutetium-177 (Lu-177) to the alpha-emitters such as Actinium-225 (Ac-225). Select presentation and publications can be found here to learn more about our targeted radiotherapy capabilities.
We are leaders in the application of Actinium-225 the potent alpha emitting radioisotope. Actinium-225 is the most powerful medical grade radioisotope known to man, yet its power is emitted over a short distance of a few millimeters making it ideal for targeted radiotherapy if it can be harnessed properly. Actinium is the leader in Ac-225 based therapies through our clinical experience, technology, intellectual property and know-how. This leadership position comes from our clinical experience where over 500 patients having been treated with our ARCs and through our clinical trials we have treated more patients with Ac-225 than any other company including our ongoing trials that are combining our Ac-225 ARCs with other therapeutic modalities such as chemotherapy and targeting agents. Learn more about our ARC clinical experience and clinical programs here.
Actinium’s Research Capabilities
Actinium is a fully integrated targeted radiotherapy development company with laboratory facilities in New York City. Actinium’s research facilities enables the efficient and timely execution of preclinical R&D to support Actinium’s internal projects as well as collaborations with industry and academic partners. Actinium's R&D efforts employ a multidisciplinary approach leveraging our team's expertise and experience in cancer cell biology, radiochemistry, radiation sciences, immunology and oncology drug development.
To learn more about Actinium’s research capabilities or discuss a collaboration please contact us at research@actiniumpharma.com.